Performance studies: definition and framework
A study undertaken to establish or confirm the performance of an IVD falls within the scope of the IVDR. Other research in which IVDs are used as part of a study (for example, to measure an endpoint), but in which the performance of the IVD itself is not investigated, falls outside the scope of chapter VI of the IVDR. These studies may fall under the scope of the Dutch Medical Research Involving Human Subjects Act [Wet Medisch-wetenschappelijk onderzoek met mensen, WMO] or the Dutch Embryo Act [Embryowet].
Definition performance study
The IVDR gives the following definition of a performance study:
The purpose of a clinical performance study is to establish or confirm aspects of clinical or analytical performance.
Analytical performance means the ability of an IVD to correctly detect or measure a specific analyte. Clinical performance means the ability to provide results that are clinically relevant.
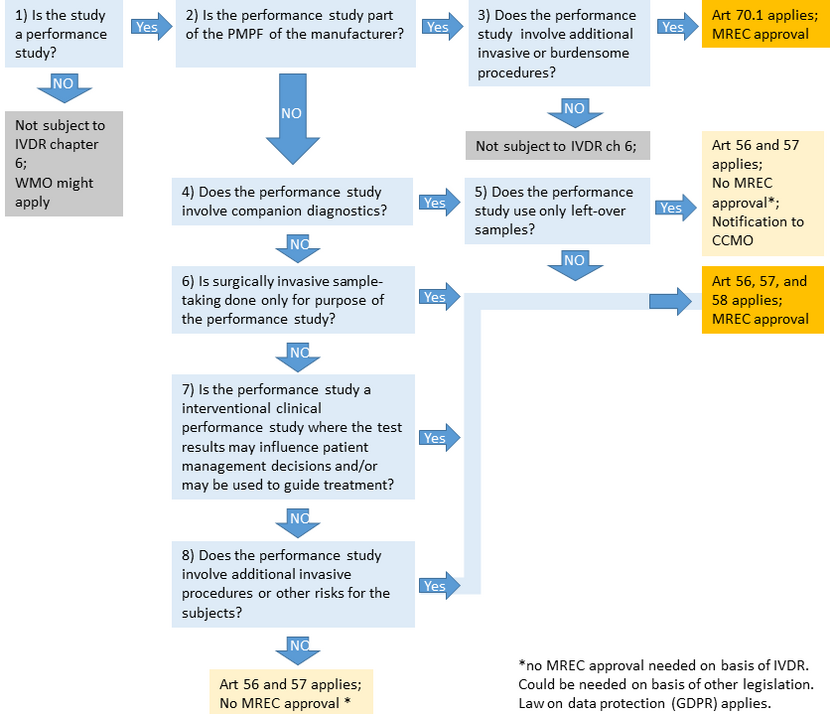
Frames for performance studies
The IVDR has the following frames for performance studies, with different requirements and procedures:
IVDR Article 58(1) and Article 70(2)
-
Performance studies involving a risk to the subject:
- in which the surgically invasive sampling is carried out solely for the purpose of the performance study;
- which is an interventional clinical performance study in which the test results may influence patient care decisions and/or be used to guide treatment, or
- in which the conduct of the study involves additional invasive procedures or other risks to the subjects of the studies.
IVDR Article 58(2)
-
Performance studies with companion diagnostics (CDx)
IVDR Article 70(1)
-
Post-market performance follow-up (PMPF) with additional invasive and/or burdensome procedures
IVDR article 57
-
Performance studies without risk to the subject. These studies do not have to be reviewed by an accredited MREC/CCMO according to IVDR.
To determine the framework that applies to your performance study, you may use the flowchart below.