Labelling IMP and AxMP
Investigational and auxiliary medicinal products should be appropriately labelled in order to ensure subject safety and the reliability and robustness of data generated in clinical trials, and in order to allow for the distribution of those products to clinical trial sites throughout the EU.
Labelling requirements for IMPs and AxMP are set out in chapter X and Annex VI of the CTR. A flow diagram for labelling under CTR is provided below.
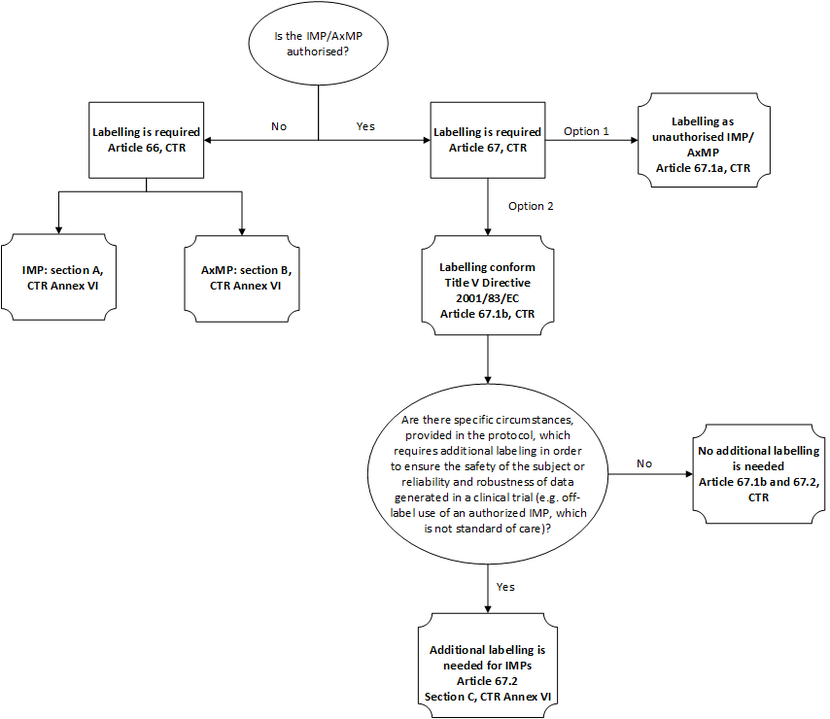
No additional labelling is needed for authorized medicinal products, unless specific circumstances are required in order to ensure subject safety and the reliability and robustness of data generated clinical trials. The specific circumstances should be outlined in the protocol. For example, an additional label is required for blinding purposes or when an authorized drug product is used in an off-label indication to a vulnerable group of patients where this form of treatment is not standard of care. An MREC or CCMO may also request an additional label, if this is considered to be necessary.
This means that no additional labelling is required for authorized medicinal product used for another indication than authorized (off-label use), if included in current treatment guidelines for this specific indication.
Labelling requirements conform CTR are not applicable for radiopharmaceuticals used as diagnostic IMP or as diagnostic AxMP. Diagnostic IMPs and diagnostic AxMPs should be labelled according GMP-Z.
There is no need to submit a mock up of the label. Only the text that is labelled on the IMP, as per Chapter X and Annex VI of the Clinical Trials Regulation, should be included in the application dossier. The language of the information on the label should be in Dutch for studies performed in the Netherlands. Multiple languages are allowed. A list of information which is to appear on the outer packaging and immediate packaging is set out in Annex VI.