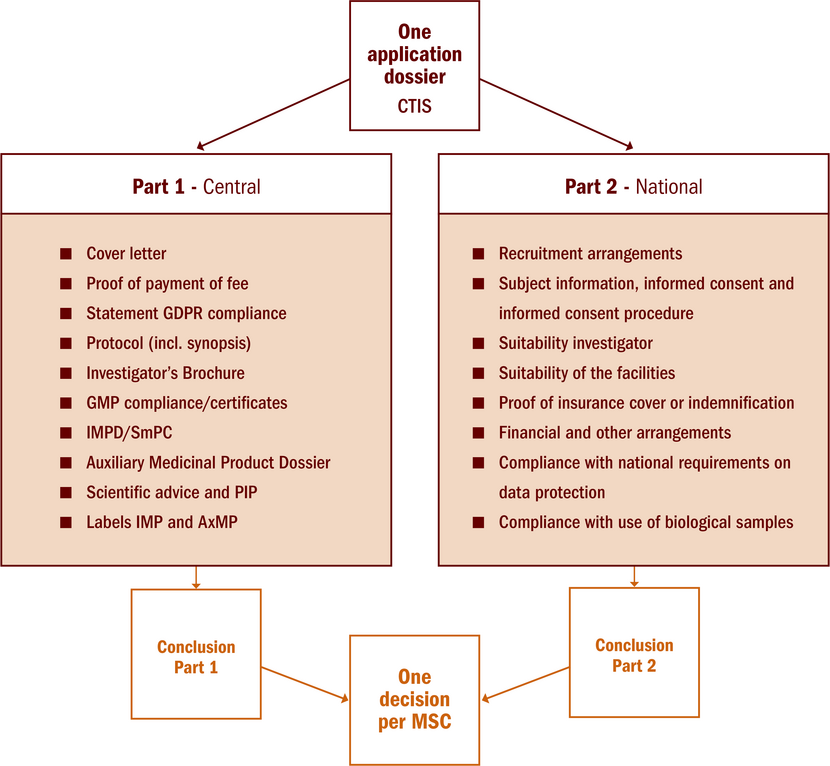
One research file: Part I and Part II
There is one single point of entry for all clinical trials conducted in the EEA: the Clinical Trial Information System (CTIS). The clinical trial application consists of part I (same documents for all MSC) and part II (national documents per MSC).
In case of multinational clinical trials, Part I application will be jointly assessed by MSC with one conclusion valid for all MSC. A reporting MS (RMS), one of the MSC, will be appointed to coordinate and consolidate the joint assessment. Part II application will be a national assessment with a conclusion only valid in this MS. Each MSC will issue a decision which is the result of the conclusion part I and part II.
A national clinical trial application also consists of a part I and a part II and is assessed by the MS concerned. This Member State is the RMS for this clinical trial by default.
See figure below with an overview of the components of part I and part II.