Validation and assessment of a substantial modification
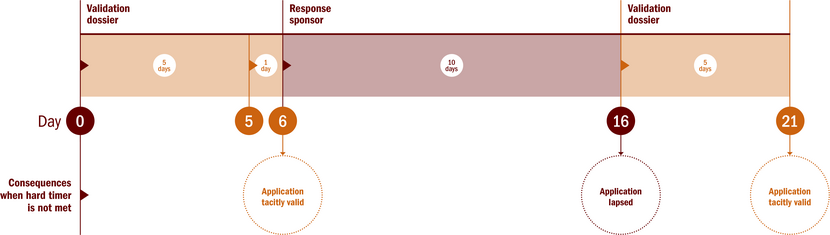
Validation of substantial modification part I
The application of a SM part I only will start with an validation by rMS. The validation phase has strict time limits. The total timeline is maximal 21 days.
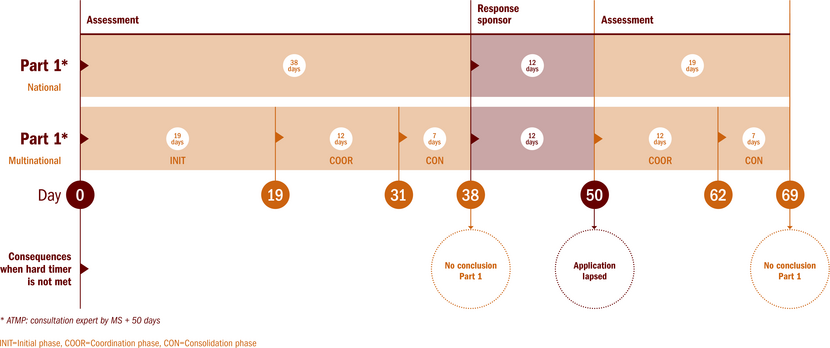
Assessment of substantial modification part I
After a positive validation, the rMS assesses the substantial modification, and draws up an assessment report within 38 days after the validation date. The rMS may request additional information from the sponsor, thereby extending the assessment period with 31 days.The assessment of a substantial modification in a multinational clinical trial will be carried out in conjunction with the MSc. In case of a low-intervention trial, the assessment also evaluates whether the clinical trial will remain that way after its substantial modification.
The assessment report contains one of the following conclusions concerning the aspects addressed in Part I of the assessment report:
- the substantial modification is acceptable;
- the substantial modification is acceptable, but subject to compliance with specific conditions which will be specifically listed in that conclusion; or
- the substantial modification is not acceptable.
Where the conclusion of the rMS is that the substantial modification is acceptable or acceptable subject to compliance with specific conditions, that conclusion shall be deemed to be the conclusion of the MSc. However, each MSc takes an individual decision and can disagree with a positive conclusion by the rMS. See the section Conclusion and opt out on which grounds the MSc can disagree with a positive conclusion. This might lead to the situation that for a given clinical trial, several versions of the part I documents exist. CTIS reflects these versions and contains both an overview of the document versions authorised at trial level and at MS level.
Where the conclusion of the rMS is that the substantial modification is not acceptable, that conclusion shall be deemed to be the conclusion of all MSc. Opt out is not possbile in this case.
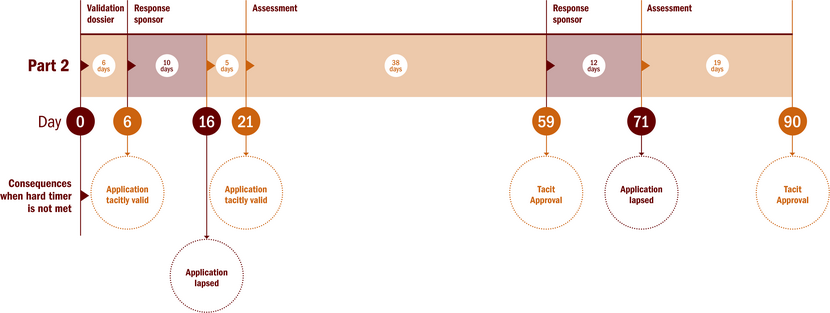
Validation and assessment of a Part II substantial modification
The MSc shall validate and assess the application and shall submit Part II of the assessment report in CTIS, including its conclusion, and the decision as to whether the substantial modification is authorised, or authorised subject to conditions, or whether authorisation is refused. The conclusion of the MSc on the SM part II is at the same time the decision regarding the SM part II. There is no separate decision phase.
During the assessment period the MSc may request, with justified reasons, additional information from the sponsor regarding the validation or assessment of the substantial modification, thereby extending the assessment period. The total duration is maximal 90 days (validation and assessment).
Substantial modification part I and part II
Where a substantial modification relates to aspects covered by Parts I and II of the assessment report, the application for authorisation of that substantial modification shall be validated in accordance with procedure of SM part I.
The aspects covered by Part I of the assessment report shall be assessed in accordance with procedure SM part I and the aspects covered by Part II of the assessment report shall be assessed in accordance with SM part II.