Validation by CCMO of clinical investigations for conformity purposes
The EU Medical Device Regulation (MDR) stipulates that clinical investigations for conformity purposes (MDR article 62/74.2) must be validated before they can be assessed by a review committee.
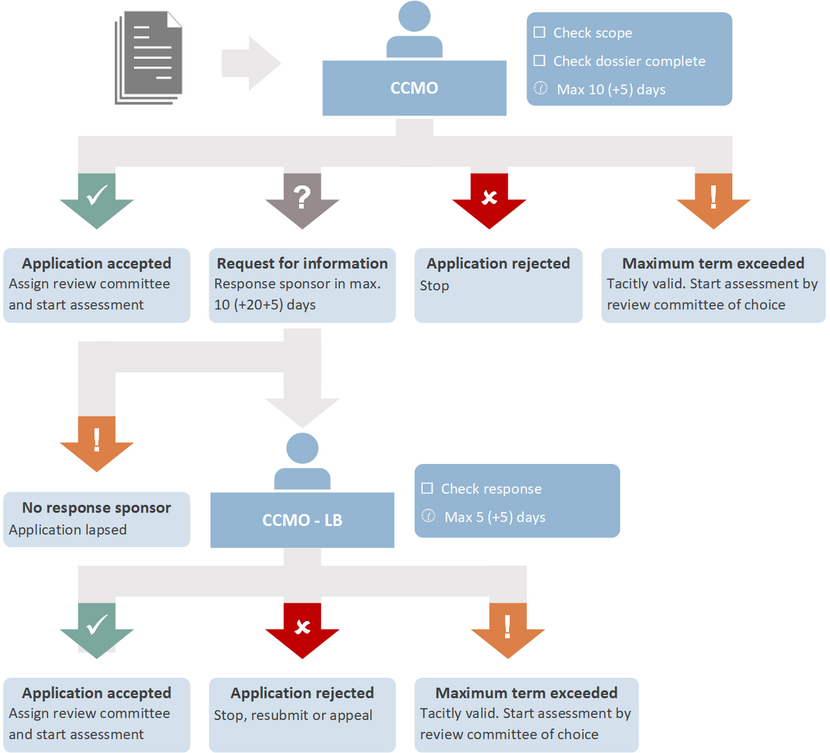
Validation of clinical investigations for conformity purposes (MDR article 62/74.2) is carried out by CCMO. CCMO checks whether the clinical investigation falls within the scope of the MDR and whether the research file is complete. The check for completeness only concerns the presence of all documents; it is not a substantive assessment.
If the clinical investigation falls within the scope of the MDR and the research file is complete, CCMO sends the file to the review committee of your choice, after which the assessment of the investigation begins. The date of a positive validation notification is the validation date.
Maximum legal deadlines apply to both the sponsor and CCMO. CCMO can extend a deadline itself or at the request of the sponsor. If the sponsor has exceeded the deadline, the submission is cancelled and the file must be re-submitted for validation. The application is tacitly validated if CCMO exceeds the maximum deadline.
In the case of a negative validation, it is possible to file an objection to CCMO. The figure below shows the procedure and the deadlines (number of calendar days and possible extension in calendar days).